Deionized Resin Regeneration

Emergency Mobile Water Treatment

Ultra - Pure Water Systems

Service + Repair

Water Softeners

Filtration
Frequently Asked Questions
Technical standards on water quality have been established by a number of organizations including the American Society for Testing and Materials (ASTM), International Organization for Standardization (ISO), United States Pharmacopoeia (USP) and the Clinical and Laboratory Standards Institute (CLSI) – previously known as the US National Committee for Clinical Laboratory Standards (NCCLS). CLSI outlines Clinical Laboratory Reagent Water (CLRW) guidelines.
ASTM Standards for Laboratory Reagent Water (ASTM D1193-91)
ASTM: American Society for Testing and Materials
Measurement (Unit) Type I Type II Type III Type IV Resistivity (MΩ-cm) > 18 > 1 > 4 > 0.2 (200KΩ) Conductivity (µS/cm) < 0.056 < 1 < 0.25 < 5 pH at 25˚C N/A N/A N/A 5.0 - 8.0 Total Organic Carbon (TOC) ppb or µg/L < 50 < 50 < 200 N/A Sodium (ppb or µg/L) < 1 < 5 < 10 < 50 Chloride (ppb or µg/L) < 1 < 5 < 10 < 50 Silica (ppb or µg/L) < 3 < 3 < 500 N/A The ASTM standards are further subdivided into A, B and C that can be used in conjunction with the type I, II, III or IV water above when bacteria levels need to be controlled.
Additional ASTM Sub-Standards for Laboratory Reagent Water
Measurement (Unit) A B C Heterotrophic Bacteria Count (CFU/ml) < 1 < 10 < 1000 Endotoxin (units per ml) < 0.03 < 0.25 N/A ISO 3696 Standard
ISO: International Organization for Standardization
Parameter Grade 1 Grade 2 Grade 3 Conductivity µS/cm (temp corrected) < 0.1 < 1 < 5 pH at 25°C N/A N/A 5.0 - 7.0 Oxidizable matter Oxygen (02) content mg/L N/A < 0.08 < 0.4 Absorbance at 254 nm and 1 cm optical path length, absorbance units < 0.001 < 0.01 N/A Residue after evaporation on heating at 110°C mg/kg N/A < 1 < 2 Silica (Si02) mg/L < 0.01 < 0.02 < N/A CLSI-CLRW Guidelines
CLSI-CLRW: Clinical and Laboratory Standards Institute – Clinical Laboratory Reagent Water
CLSI was formerly known as NCCLS (US National Committee for Clinical Laboratory Standards)Contaminant Parameter and Unit Type 3 Type 2 Type 1 CLRW Ions Resistivity (MΩ-cm) > 0.05 (50 KΩ) > 1 > 18 > 10 Organics Total Organic Carbon (TOC) ppb < 200 < 50 < 10 < 500 Pyrogens (Eu/ML) N/A N/A < 0.03 --- Particles Particles > 0.2 µm (units/mL) N/A N/A < 1 (0.22 µ filtration required) Include 0.22 µ filtration Colloids Silica (ppb) < 1000 < 100 < 10 --- Bacteria Bacteria (cfu/mL) < 1000 < 100 < 1 < 10 These values are best used as guidelines, as many applications require further treatment based on other factors. For example, many molecular biology applications require Type 1 water that is free of DNase and RNase and simple washing of instruments (usually Type 3) might require water that is pyrogen free for critical applications (Type 1).
Laboratory Water Purity Specifications ‘Consolidated’ Guidelines
Contaminant Parameter and Unit Type 1 Type 2 Type 3 Ions Resistivity (MΩ-cm) > 18 > 1 > 0.05 (50 KΩ) Silica (ppb) < 10 < 100 < 1000 Organics Total Organic Carbon (TOC) ppb < 20 < 50 < 200 Particles Particles > 0.2 µm (#/ml) < 1 N/A N/A Bacteria Particles > 0.2 µm (#/ml) < 1 < 100 < 1000 Endotoxin (Eu/ML) < 0.001 N/A N/A Typical uses for each type are outlined below:
Type 1 Required for critical laboratory applications such as HPLC, Mobile Phase Preparation, blanks and sample dilution for analytical techniques such as GC, HPLC, AA, ICP-MS, etc. Preparation of buffers and media for mammalian cell culture and IVF. Production of reagents for molecular biology applications (DNA sequencing, PCR). Preparation of solutions for electrophoresis and blotting. Type 2 Used in buffers, pH solutions and microbiological culture media preparation and for preparation of reagents for chemical analysis. Used in clinical analyzers, cell culture incubators, etc. Also used as feed water to Type 1 systems. Type 3 Used for glassware rinsing, filling autoclaves and heating baths and humidifiers. Also used as feed water to Type 1 systems. Source: www.millipore.com
USP Standards
USP: United States Pharmacopoeia
Properties USP 'Purified Water' USP 'Water for Injection' & 'Highly Purified Water' Conductivity (µS/cm @ 25°C) < 1.3 < 1.3 Total Organic Carbon (TOC) ppb or µg/L < 500* < 500* Bacteria (guideline) < 100 cfu/ml < 10 cfu/100ml Endotoxin (EU/ml) N/A 0.25 EU/ml *Or pass oxidisable substance test
Deionization (or demineralization) simply means the removal of ions. Ions are electrically charged atoms or molecules found in water that has either a net negative or positive charge. For many applications that use water as a rinse or ingredient, these ions are considered impurities and must be removed from the water. Ions with a positive charge are called “Cations” and ions with a negative charge are called “Anions”. Ion exchange resins are used to exchange non-‐desirable cations and anions with hydrogen and hydroxyl, respectively, forming
pure water (H 2 0), which is not an ion.Below is a list of ions commonly found in municipal water.
Cations
Removed by Cation Resins
Calcium (Ca++)
Magnesium (Mg++)
Iron (Fe+++)
Manganese (Mn++)
Sodium (Na+)
Hydrogen (H+)
Anions
Removed by Anion Resins
Chlorides (Cl-‐)
Sulfates (SO 4 =)
Nitrates (NO 3 =)
Carbonates (CO 3 =)
Silica (SiO 2 -‐)
Hydroxyl (OH-‐)
Reverse Osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi-permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is required to overcome the osmotic pressure.
The desalinated water that is demineralized or deionized, is called permeate (or product) water. The water stream that carries the concentrated contaminants that did not pass through the RO membrane is called the reject (or concentrate) stream.
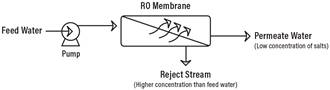
As the feed water enters the RO membrane under pressure (enough pressure to overcome osmotic pressure) the water molecules pass through the semi-permeable membrane and the salts and other contaminants are not allowed to pass and are discharged through the reject stream (also known as the concentrate or brine stream), which goes to drain or can be fed back into the feed water supply in some circumstances to be recycled through the RO system to save water. The water that makes it through the RO membrane is called permeate or product water and usually has around 95% to 99% of the dissolved salts removed from it.
It is important to understand that an RO system employs cross filtration rather than standard filtration where the contaminants are collected within the filter media. With cross filtration, the solution passes through the filter, or crosses the filter, with two outlets: the filtered water goes one way and the contaminated water goes another way. To avoid buildup of contaminants, cross flow filtration allows water to sweep away contaminant build up and also allow enough turbulence to keep the membrane surface clean.
RO membranes will inevitably require periodic cleaning, depending on the feed water quality. As a general rule, if the normalized pressure drop or the normalized salt passage has increased by 15%, then it is time to clean the RO membranes. If the normalized permeate flow has decreased by 15% then it is also time to clean the RO membranes. You can either clean the RO membranes in place or have them removed from the RO system and cleaned off site.
Dealkalizers are most often used as pre-treatment to boiler and are usually preceded by a water softener. Alkalinity is a factor that most often dictates the amount of boiler blowdown. High alkalinity promotes boiler foaming and carryover and causes high amounts of boiler blowoff. When alkalinity is the limiting factor affecting the amount of blowdown, a dealkalizer will increase the cycles of concentrations and reduce blowdown and operating costs.
The reduction of blowdown by dealkalization keeps the water treatment chemicals in the boiler longer, thus minimizing the amount of chemicals required for efficient, noncorrosive operation.
Carbonate and bicarbonate alkalinities are decomposed by heat in boiler water releasing carbon dioxide into the steam. This gas combines with the condensed steam in process equipment and return lines to form carbonic acid. This depresses the pH value of the condensate returns and results in corrosive attack on the equipment and piping.
In general, a dealkalizer is best applied to boilers operating below 700 psi (48 bar). In order to justify installation of a dealkalizer on low-pressure boilers, the alkalinity content should be above 50 ppm with the amount of make-up water exceeding 1,000 gallons (approx. 4,000 litres) per day
Water Softener is an ion exchange system that works by removing calcium ions and magnesium ions that make up hard water.
When water flows through a Softener, the system exchanges these hard water minerals, and the softened water then leaves the water softening system.
Water is very important for the operation of many businesses. If you’re the owner of a business or commercial facility that relies on water for its day-to-day functions, it’s important that you understand the difference between hard and soft water. This will help you know which type of water is best suited for your commercial operations.
There’s no doubt that installing a commercial water softener is a great investment for any business owner. However, it is always important to understand the difference between soft and hard water. Hard water has a lot of minerals such as calcium and magnesium. These minerals can affect the quality of your facility’s appliances when they build up over time. Soft water doesn’t have these minerals.
Hausers Water Systems can provide preventative maintenance needs to meet your schedule. Complete Water Treatment solutions with affordable preventative maintenance service tailored to meet your business requirements. Whether you need your Water Softening system checked every six months or have a medical water treatment system in need of monthly inspection, we are ready to keep your system running at your standard